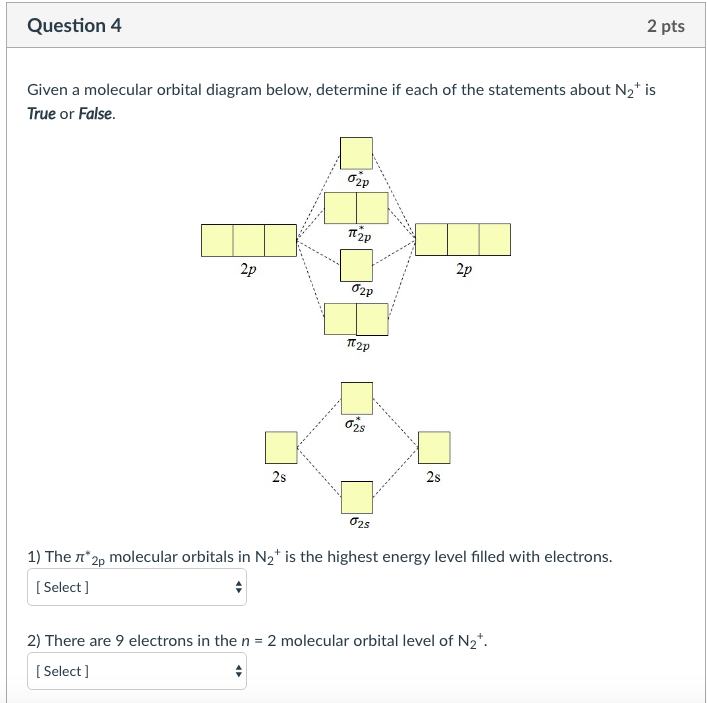
40 n2+ molecular orbital diagram
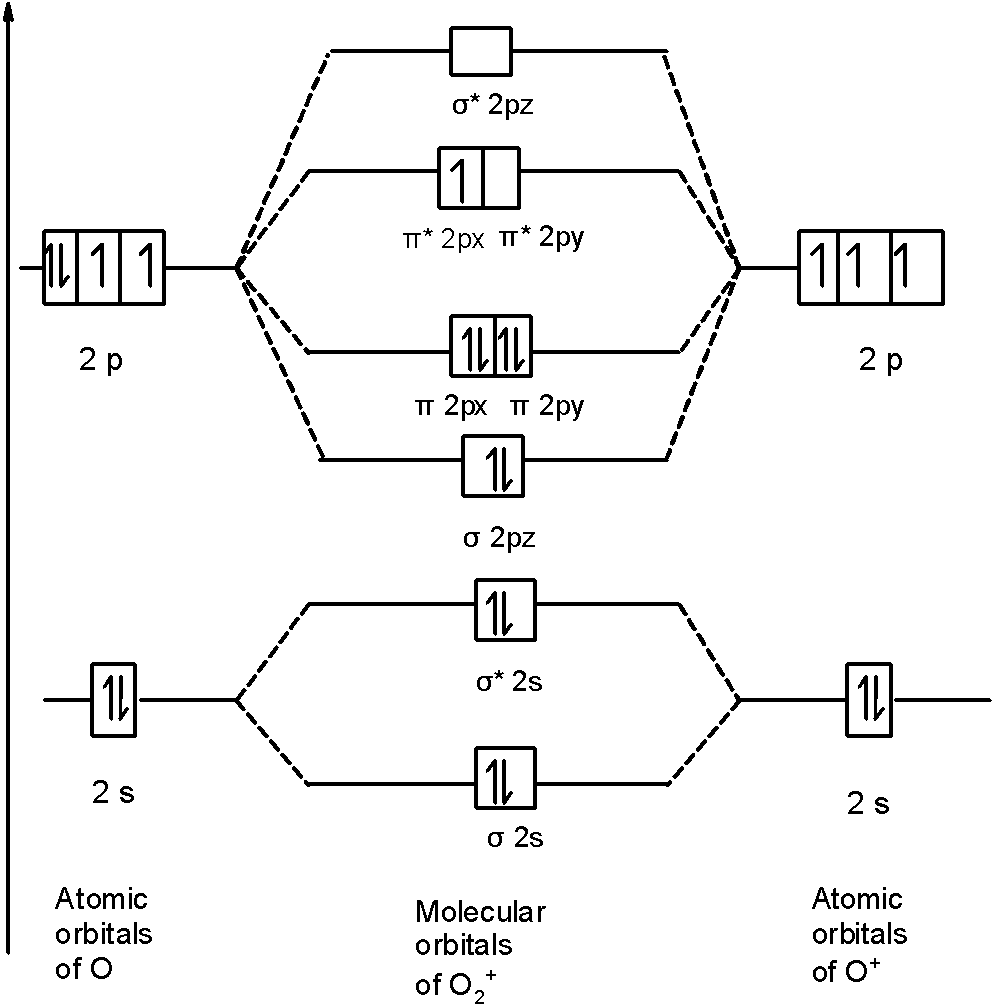
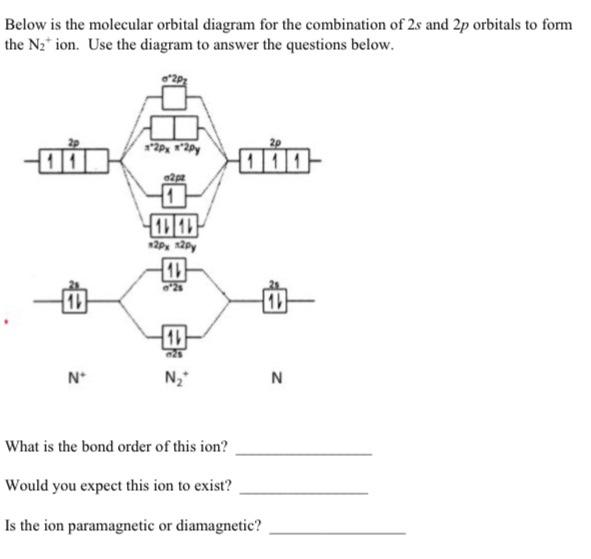
Solved Construct the molecular orbital diagram for N2 and ... Best Answer 100% (39 ratings) Transcribed image text: Construct the molecular orbital diagram for N2 and then identify the bond order Bond order 0.5 O 1.5 O 2.5 2s 2s Click within the blue boxes to add electrons. Previous question Next question write the molecular orbital diagram of n2 and calculate ... The total number of electrons present in the N 2 molecule is 14. N 2+ ion is formed by the loss of one electron from the N 2 molecule. This lost electron will be lost from σ (2p z) orbital. Hence, the electronic configuration of N 2+ ion will be N 2+ = KK [σ (2s)] 2 [σ* (2s)] 2 [π (2p x )] 2 [π (2p y )] 2 [σ (2p z )] 1 Here, N b =7, N a =2 so that
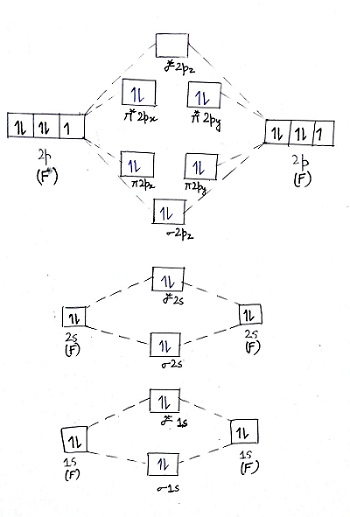
Molecular Orbital Diagram Ne2 - schematron.org Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2.

N2+ molecular orbital diagram
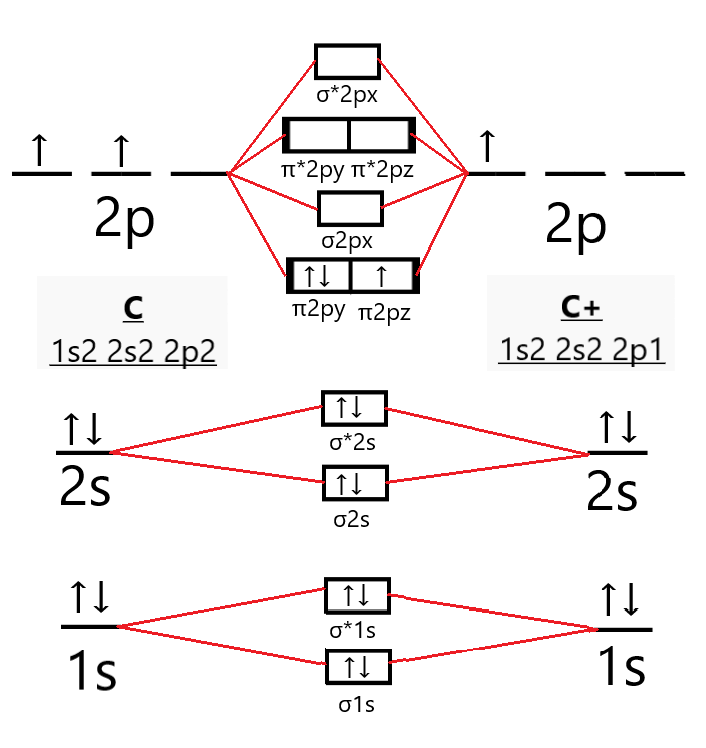
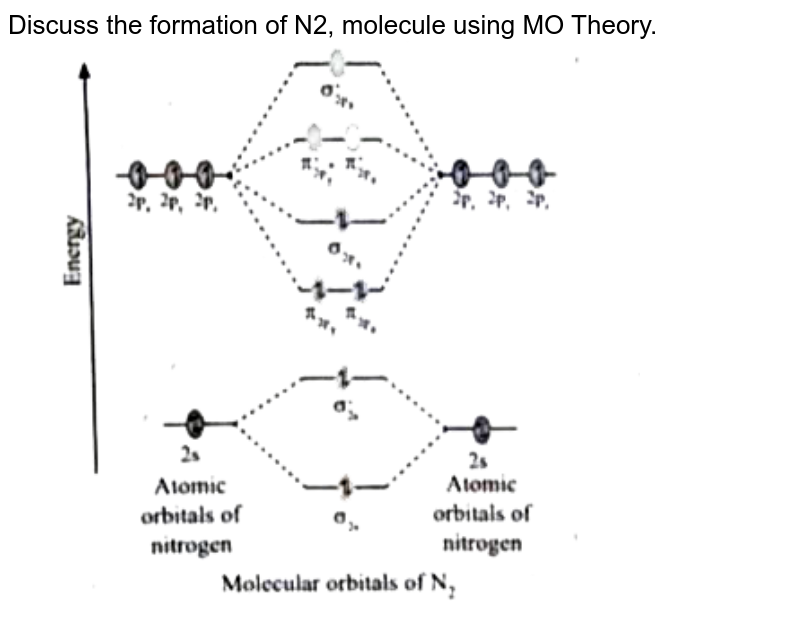
MO Diagram for N2+ (Molecular Orbital) lesson plan | Spiral MO Diagram for N2+ (Molecular Orbital) - lesson plan ideas from Spiral. mo-diagram-for-n2-molecular-orbital. Welcome to Clip from Interactive video lesson plan for: MO Diagram for N2+ (Molecular Orbital) Activity overview: There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc). ... MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Draw the molecular orbital diagram of N2N2 + N2 Write ... Basic structure of molecular orbital diagram for nitrogen is: Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents the second atom of nitrogen molecule. Atomic number of nitrogen is seven. Therefore in N 2 there are a total fourteen electrons.
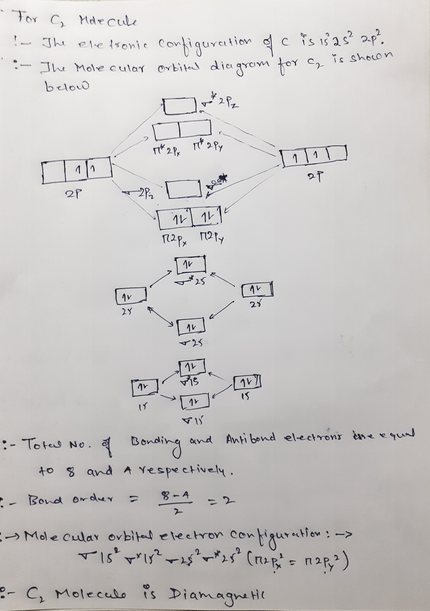
N2+ molecular orbital diagram. Draw the molecular orbital diagram of N2 , N2^ + N2 ... Question Draw the molecular orbital diagram of N 2 , N 2+ N 2− . Write their electronic configuration, find the bond order and predict their magnetic behaviour. Arrange the above in increasing order of bond length. Hard Solution Verified by Toppr Bond order = 21 [N b −N a ] N b = number of bonding electrons N a = number of anti-bonding electrons Solved 5. Draw the molecular orbital diagram for N2. Label ... Draw the molecular orbital diagram for N2. Label all of the atomic orbitals and molecular orbitals and put the correct number of electrons in. You do not need to draw the shapes of any of the orbitals. a) MO diagram b) Based on your MO diagram, is N2 diamagnetic or paramagnetic? c) Calculate the bond order for N2. Question: 5. N2+ Mo Diagram - schematron.org Feb 03, 2019 · In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory – . Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.M.O. diagram for N2+Molecular orbital diagram - Wikipedia 3 thoughts on “ N2+ mo diagram ” Give the molecular orbital energy diagram of N2 and O2 ... Molecular orbital energy level diagram (MOED) of 'N 2 ' :. Electronic configuration of nitrogen (Z = 7) is 1s 2 2s 2 2p 3.Since nitrogen atom has 7 electrons, the molecular orbitals of nitrogen molecule (N 2) has 14 electrons which are distributed as below :. Molecular orbital energy level diagram of N 2 molecule • Bond order = (8 2)/2 = 3 (N ≡ N)
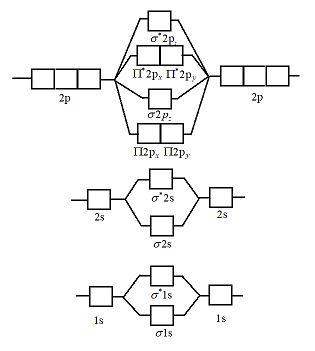
Molecular Orbital Diagram For Ne2 We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. Leave a Comment Cancel reply - BYJUS Bond order formula is given as below Bondorder=1/2 [a-b] where a = Number of electrons in bonding molecular orbitals. b = Number of electrons in antibonding molecular orbitals. (i) Structure of N 2 Electronic configuration of N 2 (14 electrons) = (σ1s) 2 (σ*1s) 2 (σ2s) 2 (σ*2s) 2 (π) 4 (2p z) 2 a = 10 b= 4 Bond order = 1/2 (10 - 4) Bond order = 3 In the molecular orbital diagram for the molecular ion, N2+, JEE Main 2018: In the molecular orbital diagram for the molecular ion, N2+, the number of electrons in the σ2p molecular orbital is: (A) 0 (B) 1 (C) N2+ Mo Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram.
Draw molecular orbital diagram for N(2)^(+) molecule. With the help of molecular orbital theory, draw the molecular orbital energy level diagram for `N_ (2)` molecule. Also calculate the bond order and predict the magnetic behaviour. 644941652 900+ 19.1 k+ Draw the molecular orbital diagram for oxygen molecule ` (O_2)`. 646031874 700+ 7.4 k+ 10:10 Molecular orbitals in Nitrogen - ChemTube3D There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds. Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. How is the molecular orbital diagram of N2 determined? - Quora Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma anti bond orbital
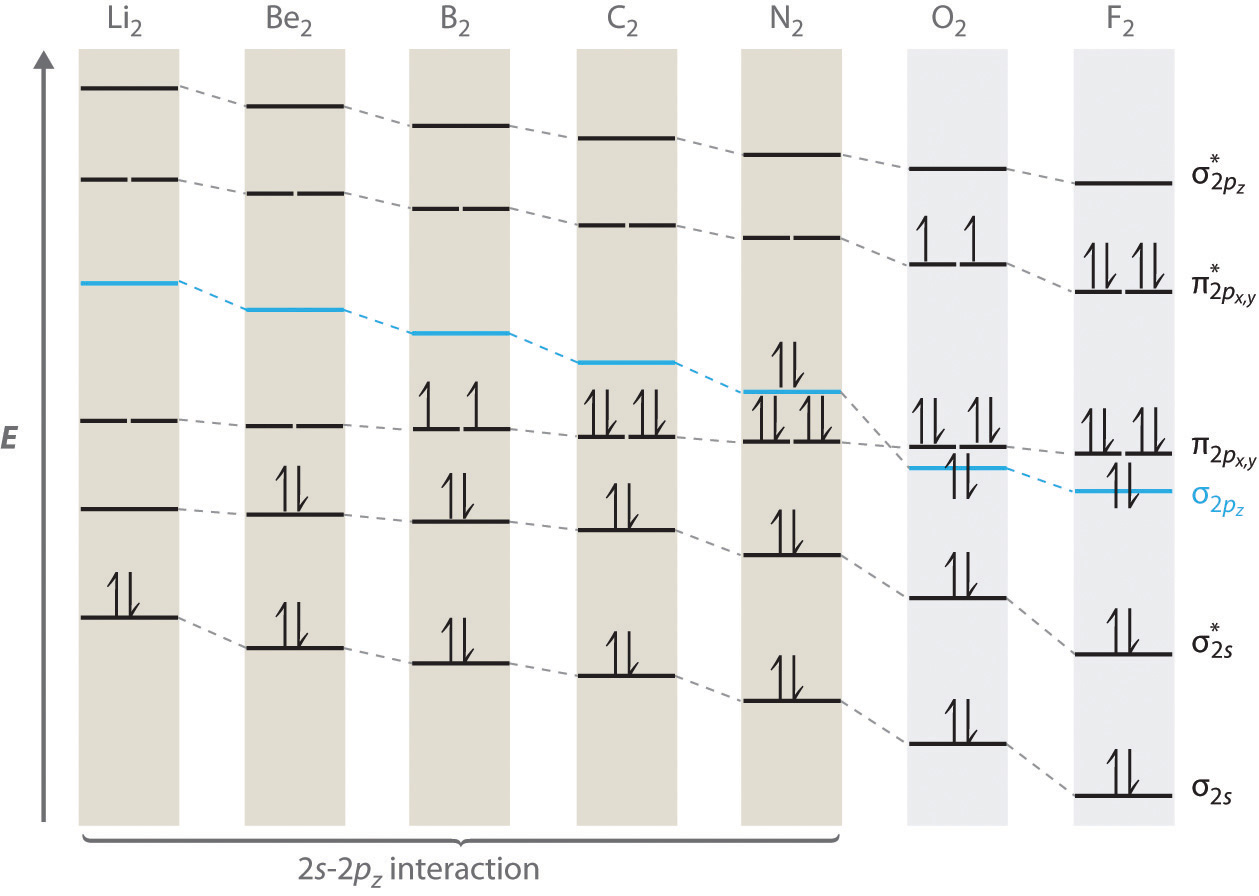
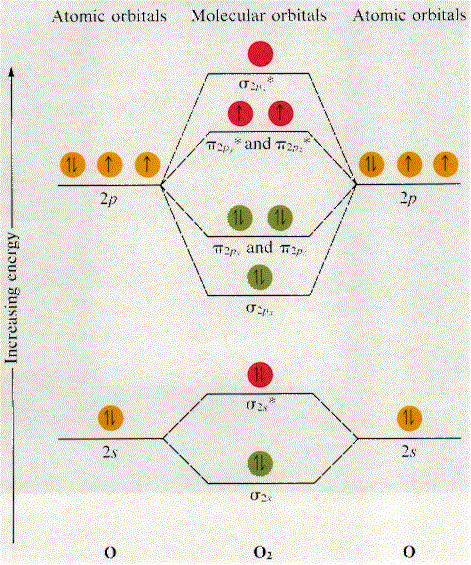
Why is the molecular orbital diagram for O₂ different from ... Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
Molecular Orbitals for N2 - Newcastle University Molecular Orbitals for N2. Jmol models of calculated wavefunctions. To view a model, click on a molecular orbital in the energy level correlation diagram shown Mouse Control of Models. Left mouse drag to rotate; Shift Left drag up or down to resize; Shift Right drag or Shift Left drag horizontally to z-rotate; Right click for menu Notes Usage
What are the molecular orbital configurations for N_2^+, N ... Organic Chemistry Hybridization and Atomic and Molecular Orbitals Molecular Orbitals and Hybridizations. 1 Answer Truong-Son N. Nov 2, 2015 If we build the MO diagram for #"N"_2#, it looks like this: First though, notice that the #p# orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be.
[Solved] 1. Write the molecular orbital diagram for the ... 1. Write the molecular orbital diagram for the molecular ion, N2+. Determine the number of. 2. What is the bond order in O2+? _______. 3. Draw the molecular orbital diagram for B2. The number of unpaired electrons in the.
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of N 2 . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior:
Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Medium Solution Verified by Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b −N a ]/2=[10−6]/2=2.
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
N2 Lewis Structure, Molecular Geometry, and Hybridization ... For more detailed knowledge you can refer to the polarity of N2. Molecular Orbital Diagram of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
N2 Lewis Structure: Full Guide (2022 Updated) Each molecule has its electron configuration consisting of a sigma bond and a pi bond, known as molecular orbitals. The molecular orbital theory determines the stability order, magnetic nature, and the number of bonds in a molecule. The configuration of N2 is 1S2, *1S2, 2S2, *2S2, 2Px2, 2Py2, 2Pz1, according to the energy level diagram. FAQS
Molecular orbital (MO) diagram for N2 and N2^- I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For N X 2 the orbitals in increasing energy are: σ 1 s < σ 1 s ∗ < σ 2 s < σ 2 s ∗ < π 2 p x, π 2 p y < σ 2 p z < π 2 p x ∗, π 2 p y ∗ < σ 2 p z ∗ because it has 14 electrons.
Draw the molecular orbital diagram of N2N2 + N2 Write ... Basic structure of molecular orbital diagram for nitrogen is: Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents the second atom of nitrogen molecule. Atomic number of nitrogen is seven. Therefore in N 2 there are a total fourteen electrons.
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
MO Diagram for N2+ (Molecular Orbital) lesson plan | Spiral MO Diagram for N2+ (Molecular Orbital) - lesson plan ideas from Spiral. mo-diagram-for-n2-molecular-orbital. Welcome to Clip from Interactive video lesson plan for: MO Diagram for N2+ (Molecular Orbital) Activity overview: There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc). ...





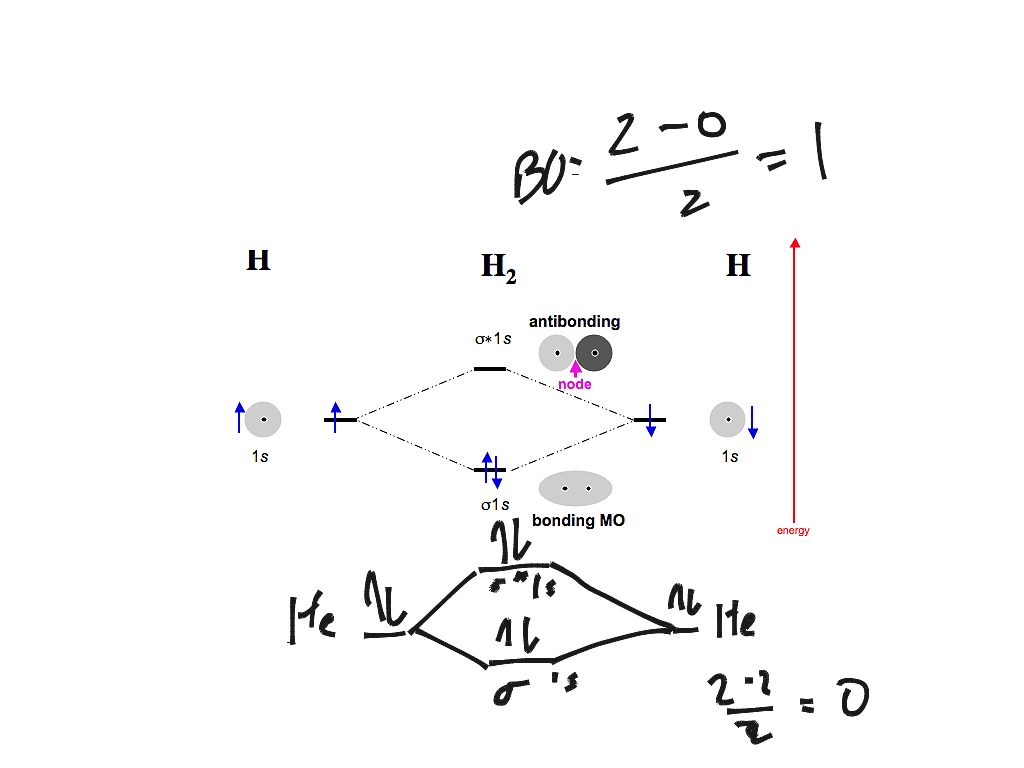















0 Response to "40 n2+ molecular orbital diagram"
Post a Comment