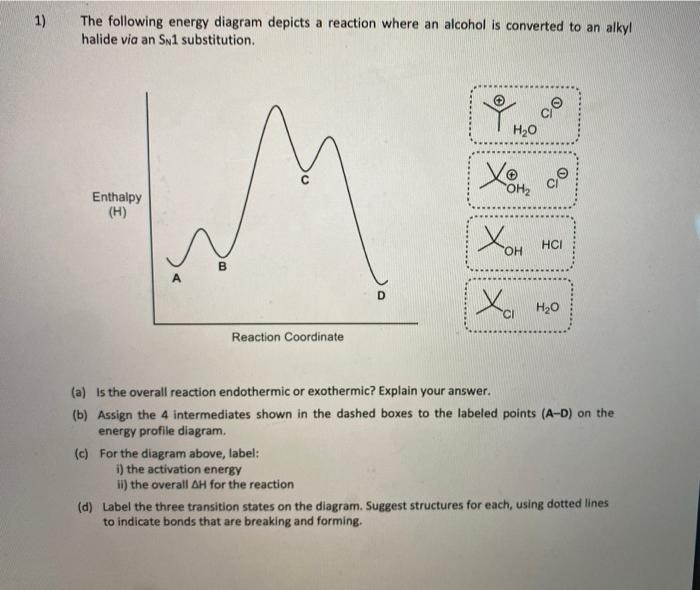
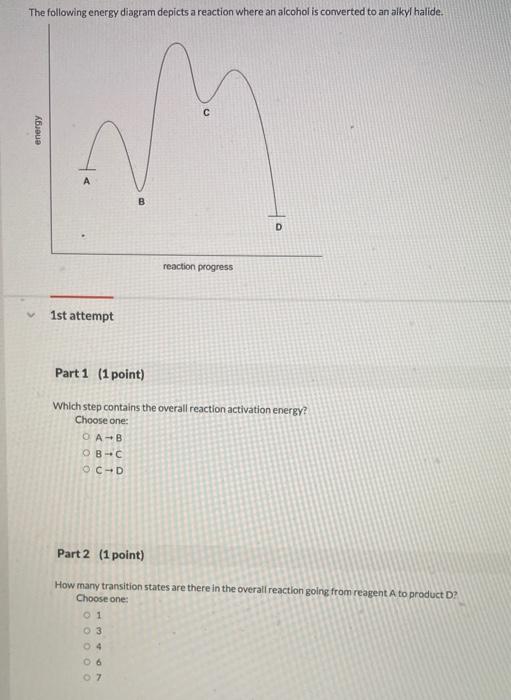
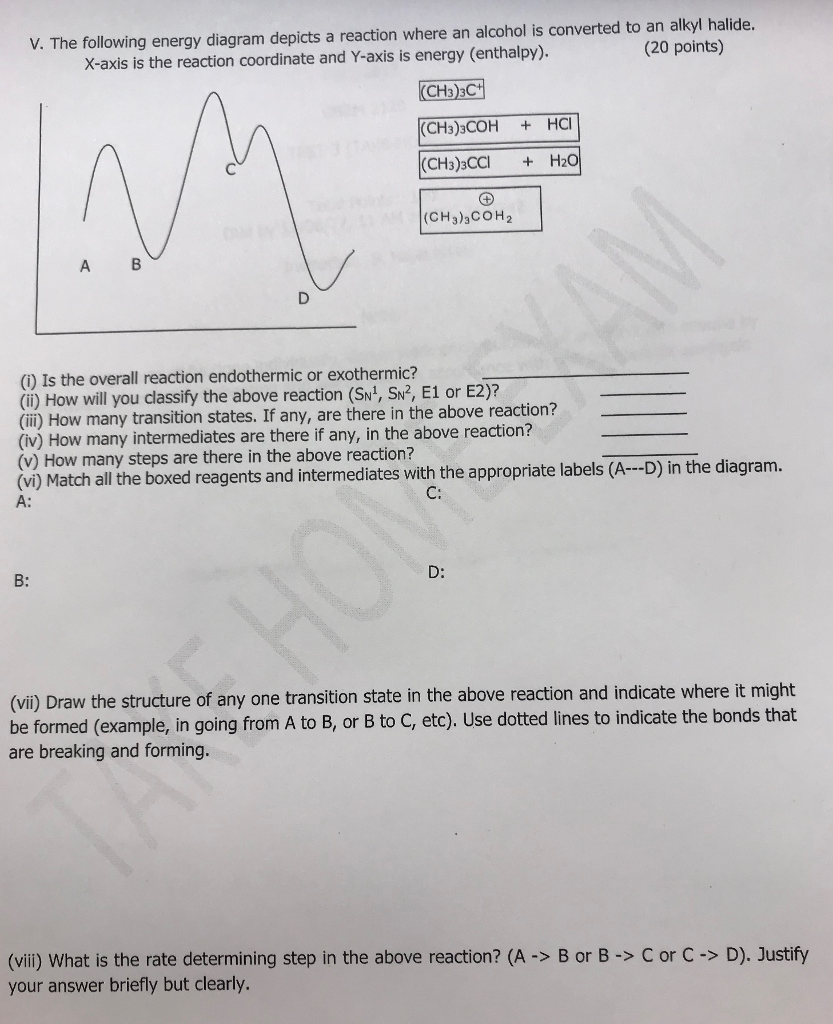
38 the following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide
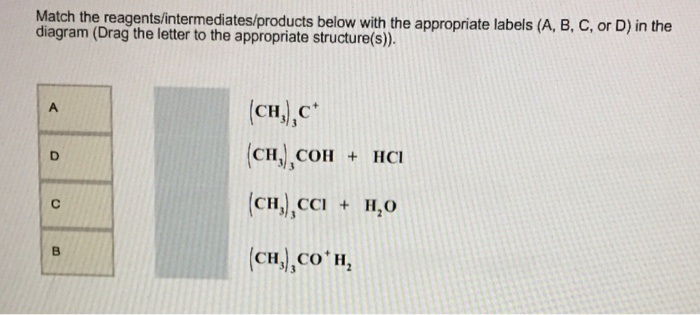
Chemistry questions and answers. The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. Match the reagents/intermediates/products below with the appropriate labels (A, B, C, or D) in the diagram (Drag the letter to the appropriate structure (s)). The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. x-axis is the reaction coordinate and Y-axis is energy (enthalpy). (20 points) (CH3)3COH +HCI (CH3)3CCI H2O (CH3)3COH2 () Is the overall reaction endothermic or exothermic?
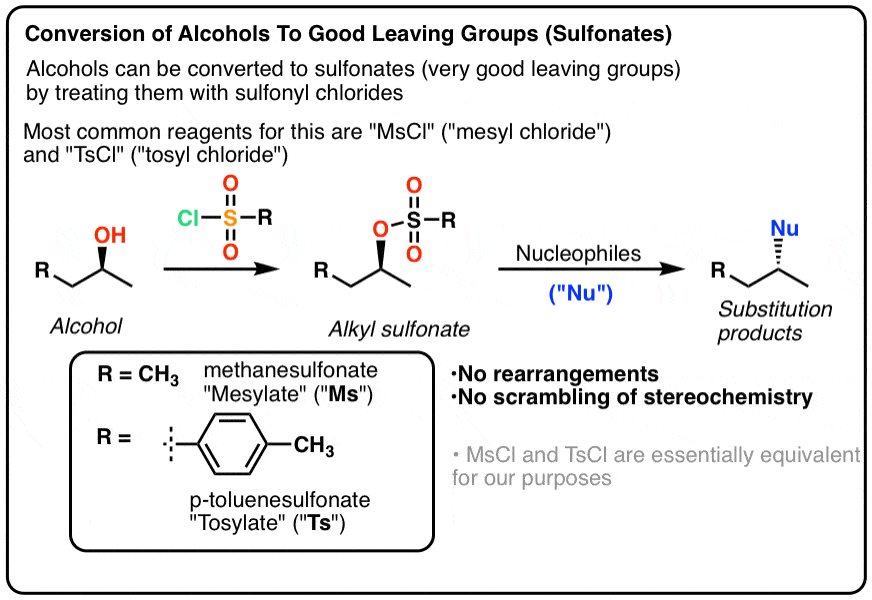
Complete the Following Reactions: Hydrolysis of Esters. The reverse reaction of ester formation can be used to breakdown esters into a carboxylic acid and an alcohol. This reactions requires the incorporation of water into the ester linkage, and is thus called a hydrolysis reaction.

The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide
Addictive Alkaloids. Since ancient times, plants have been used for medicinal purposes. One class of substances, called alkaloids, found in many of these plants has been isolated and found to contain cyclic molecules with an amine functional group.These amines are bases. They can react with H 3 O + in a dilute acid to form an ammonium salt, and this property is used to extract them from the plant: Alkyl halide or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms (Fluorine, chlorine, bromine or iodine). They can also be manufactured from any organic precursors such as alkanes, alkenes, or alcohols and carboxylic acids. Generally, alkyl halides contain hydrogen atoms attached to the sp ... The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by ...
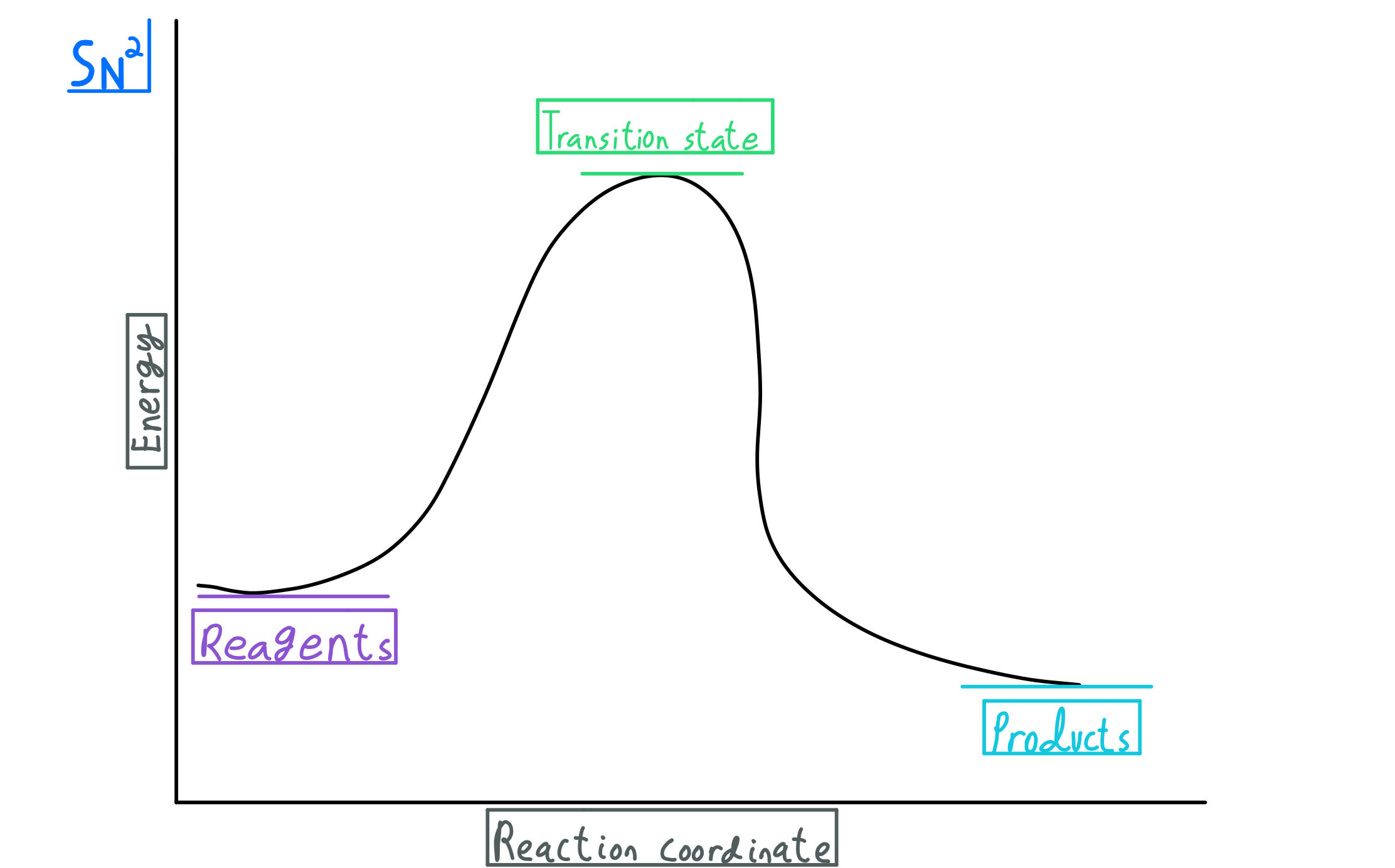
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. Reaction Coordinate Diagram of an S N 2 Reaction. The reaction of hydroxide ion with chloromethane occurs in a single step. The activation energy, E a, reflects the stability of the transition state, which depends upon the structure of the substrate, the nucleophile, and the leaving group. Chloroethane is the simplest and least toxic member of the class of chloroethanes, that is ethane in which a single hydrogen is substituted by a chlorine.A colourless gas at room temperature and pressure (boiling point 12℃), it is used as a mild topical anaesthetic to numb the skin prior to ear piercing, skin biopsies, etc., and is also used in the treatment of sports injuries. 1-Bromopropane is a colorless liquid. Commercial 1-bromopropane includes not only 1-bromopropane, but also additives that improve its performance in the desired application and stabilizers to inhibit decomposition.1-Bromopropane was originally used in the production of pesticides, flavors and fragrances, pharmaceuticals, and other chemicals. rxn of an alkyl halide w/ ammonia leads to primary, secondary, tertiary amines, and even quaternary ammonium ions. In contrast, the Gabriel synthesis and the rxn of an alkyl halide w/ azide ion form only primary amines.
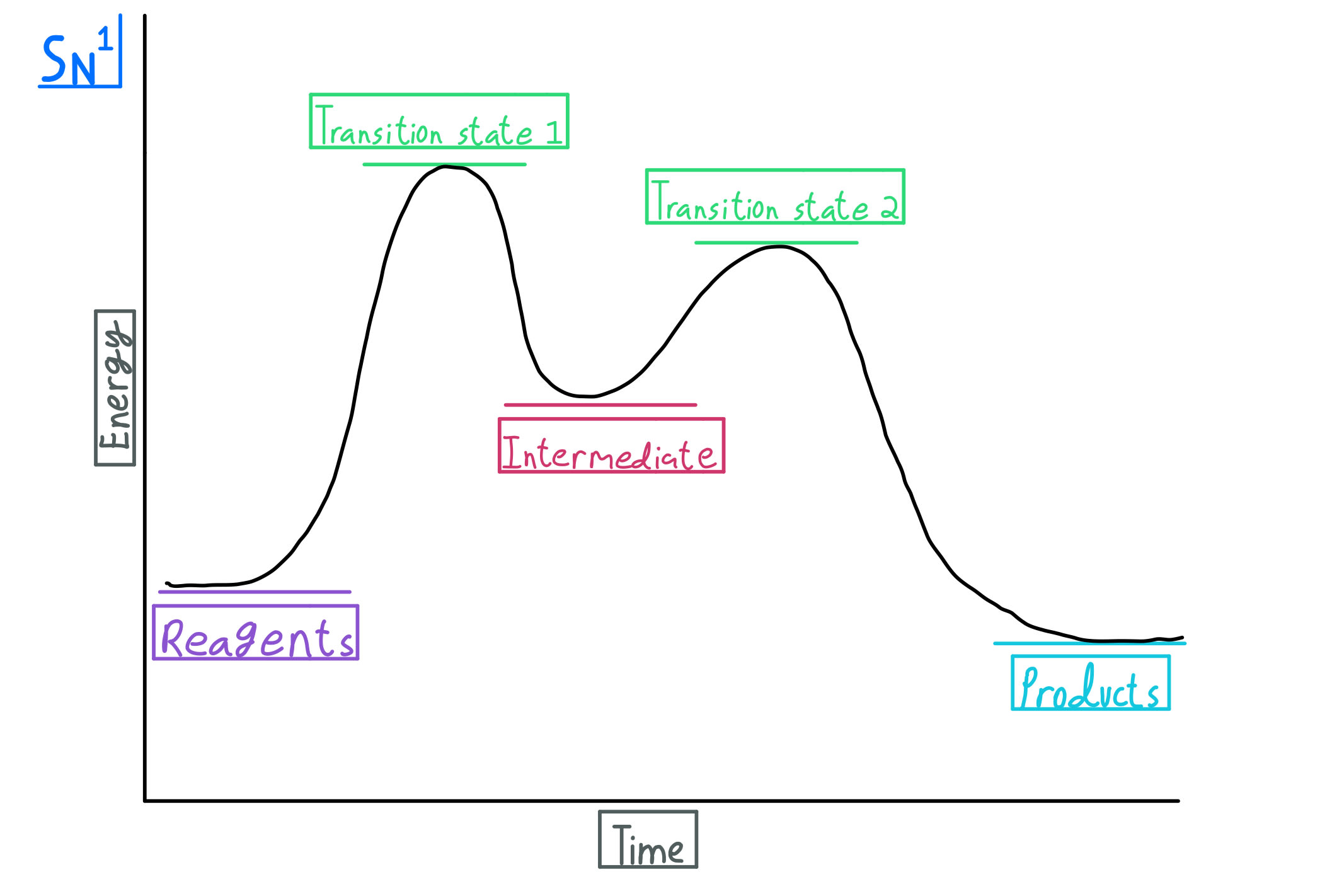
Minnesota State University Moorhead Transcribed image text: 1) The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide via an Sw1 substitution. In the presence of a strong acid, an alcohol can be dehydrated to form an alkene. The acid used in this experiment is 85% phosphoric acid and the alcohol is cyclohexanol. The phosphoric acid is a catalyst and as such increases the rate of reaction but does not affect the overall stoichiometry. It can be seen from the balanced reaction The following reactions are all examples of decarboxylation (loss of CO 2). In the first, bromine replaces the carboxyl group, so both the carboxyl carbon atom and the remaining organic moiety are oxidized. Silver salts have also been used to initiate this transformation, which is known as the Hunsdiecker reaction. The second reaction is an ...
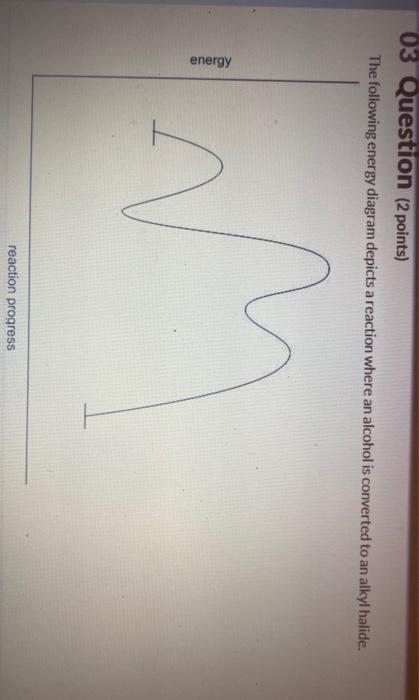
Transcribed image text: 03 Question (2 points) The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. energy ... SolutionS. The alkyl group (CH 3 CH 2 CH 2 –) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane. The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride. Thionyl chloride is preferred because in this reaction alkyl halide is formed along with gases SO 2 and HCl. The two gaseous products are escapable, hence, the reaction gives pure alkyl halides. The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. (CH3)3C (CH3) COH HCI (CH3)3 CCI H20 (CH3) COH Reaction progress O exothermic is the overall reaction exothermic or endothermic? O endothermic ; Question: The following energy diagram depicts a reaction where an alcohol is converted to an alkyl ...
a. the alkyl halide is impure b. hydrolysis, a competing reaction, is also taking place c. the initially formed product rearranges d. the ethanol used as a solvent contains a small percentage of water e. the alkyl halide contains three different types of hydrogens that can be attacked by the base

Us20170114196a1 Combined Material System For Ion Exchange Membranes And Their Use In Electrochemical Processes Google Patents
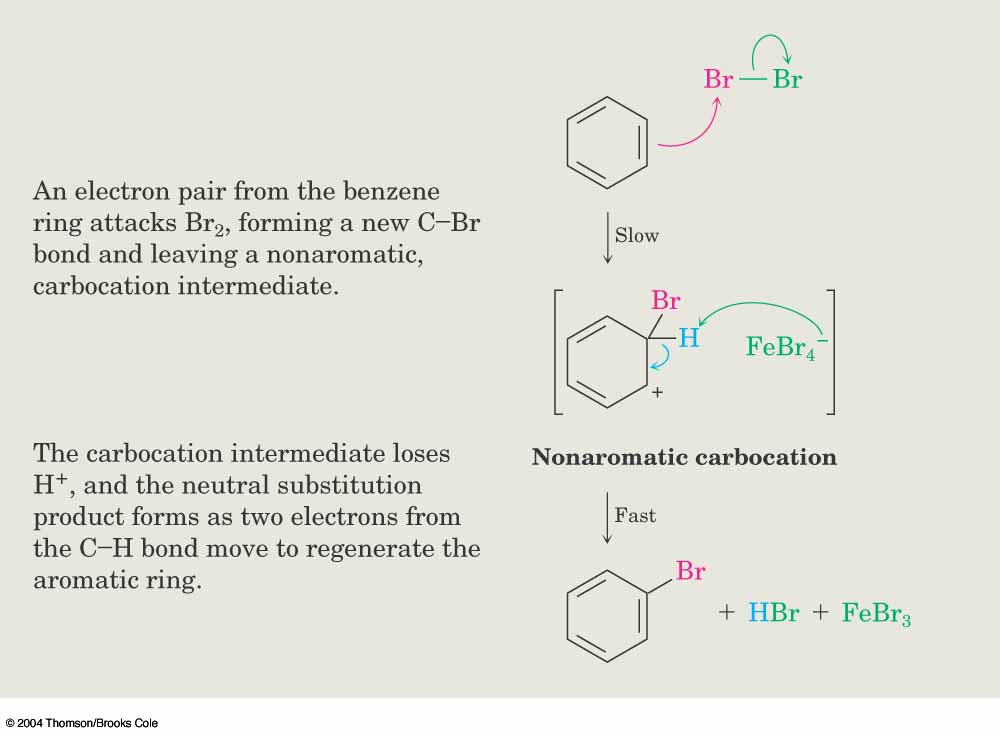
16.4 ELECTROPHILIC AROMATIC SUBSTITUTION REACTIONS OF BENZENE 755 Step 2 Reaction of the benzene p electrons with the electrophile to form a carbocation inter- mediate. (Notice that either of the oxygens can accept the electron pair.) Step 3 Loss of a proton from the carbocation to give a new aromatic compound. Nitration is the usual way that nitro groups are introduced into aromatic rings.
Answer to Solved Question 4 of 19 (1 point) The following energy. Science; Chemistry; Chemistry questions and answers; Question 4 of 19 (1 point) The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide.
The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. a) Is the overall reaction endothermic of exothermic? b) Match A, B, C, and D to the boxes. c) Which intermediate is the most unstable? d) Is this reaction similar to SN2 or SN1, and why? e) Which step is the slowest? A to B, B to C, or C to D, and ...
Infrared Spectroscopy 1. Introduction The light our eyes see is but a small part of a broad spectrum of electromagnetic radiation. On the immediate high energy side of the visible spectrum lies the ultraviolet, and on the
The alkyl hydrogen sulfate can be converted to an alcohol by boiling in water. This proceeds usually by S N 1 substitution where water is the nucleophile and bisulfate is the leaving group. The product has the same regiochemistry as an alcohol formed by direct hydration of the same alkene. (Markovnikov orientation).
In the reaction of 2-bromobutane in a solution of sodium ethoxide and ethanol, the products will be 2-butene and 1-butene in a ratio of approximately 7:3. Essentially the opposite of Markovnikoff addition, it allows for the prediction of chemical products upon the elimination reaction of an alkyl halide.
Transcribed image text: 03 Question (1 point) The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. energy.

Inorganic Electron Transport Materials In Perovskite Solar Cells Lin 2021 Advanced Functional Materials Wiley Online Library
Solution for The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide.
Both reactions will involve the initial protonation of the alcohol to form an oxonium ion. Then, the loss of the water leaving group will yield a tertiary carbocation. Where the reactions differ is that the chloride anion is a good nucleophile, whereas the conjugate base of sulfuric acid is essentially non-nucleophilic.
Nevertheless, during the marking process following the little syllabus guidance, examiners did allow the use of any one of the elimination mechanisms and thus, for example, did not penalize the use of an E1 mechanism for the dehydration reaction of a primary substrate/alcohol (this is of course not what we want to see as an answer)."

Bimetallic Oxidative Addition Involving Radical Intermediates In Nickel Catalyzed Alkyl Alkyl Kumada Coupling Reactions Journal Of The American Chemical Society
Reactions of Substituent Groups. 1. Oxidation of Alkyl Side-Chains. The benzylic hydrogens of alkyl substituents on a benzene ring are activated toward free radical attack, as noted earlier. Furthermore, S N 1, S N 2 and E1 reactions of benzylic halides, show enhanced reactivity, due to the adjacent aromatic ring.
spectrophotometer, they encounter an energy source. For the purposes of this chapter, the source is an electron impact tungsten filament at 70 eV (Section 1.5.1.2a). Energy emitted from the source removes a single electron from a sample molecule. This is the most basic reaction and is illustrated by methanol below (Figure 2.1). e- + CH 3OH ...
let's look at a few nucleophilic substitution reactions of alcohols and I'm assuming you've seen an sn1 and sn2 mechanism before let's start with a primary alcohol so this is ethanol it's a primary alcohol because the carbon bonded to the O H is bonded to one other carbon and primary alcohols react with hbr to form an alkyl bromide via an sn2 process and so we have HBR our strong acid and we ...
Transcribed image text: The following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide. energy А B D reaction progress 1st ...

Solar Fuels And Feedstocks The Quest For Renewable Black Gold Energy Environmental Science Rsc Publishing
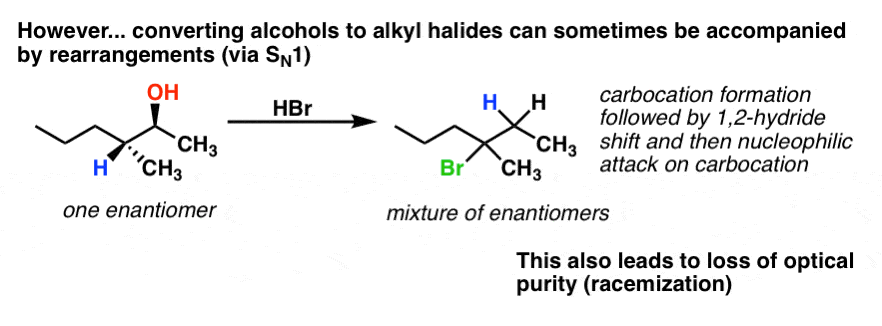
This lab experiment proposes the synthesis of an alkyl halide by reacting the corresponding alcohol with a hydrogen halide in an easy and inexpensive SN1 reaction. 1,2 tert-Butanol reacts readily with HCl and forms the corresponding tert-butyl chloride at room temperature. SN1 mechanisms are unimolecular because its slow step is unimolecular ...
8.5. Elimination reactions. Alkyl halides undergo elimination via two common mechanisms, known as E2 and E1, which show some similarities to S N 2 and S N 1, respectively. In E2, elimination shows a second order rate law, and occurs in a single concerted step (proton abstraction at C α occurring at the same time as Cβ-X bond cleavage).
The S N 2 Reaction Notes: In the SN2 reaction, the nucleophile attacks from the most δ+ region: behind the leaving group. This is called a back-side attack. This back-side attack causes an inversion (study the previous slide): after the leaving group leaves, the other substituents shift to make room for the newly-bonded nucleophile, changing the stereochemistry of the molecule.

Metal Halide Perovskite Nanocrystals Synthesis Post Synthesis Modifications And Their Optical Properties Abstract Europe Pmc
One key message you want to remember is to NEVER do an S N 2 on a tertiary alkyl halide. The carbon is simply too sterically hindered (crowded) and cannot be attacked by the nucleophile. The Nucleophile in S N 2 reactions. At some point in your organic 1 class you will need to determine if the reaction goes by S N 1 or S N 2 reaction.
The Grignard reaction is an organic reaction used to produce a variety of products through the reaction of an organomagnesium compound, also known as an electrophilic "Grignard reagent," followed by an acidic reaction. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism.
The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by ...
Alkyl halide or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms (Fluorine, chlorine, bromine or iodine). They can also be manufactured from any organic precursors such as alkanes, alkenes, or alcohols and carboxylic acids. Generally, alkyl halides contain hydrogen atoms attached to the sp ...
Addictive Alkaloids. Since ancient times, plants have been used for medicinal purposes. One class of substances, called alkaloids, found in many of these plants has been isolated and found to contain cyclic molecules with an amine functional group.These amines are bases. They can react with H 3 O + in a dilute acid to form an ammonium salt, and this property is used to extract them from the plant:

Advanced Photonic Processes For Photovoltaic And Energy Storage Systems Sygletou 2017 Advanced Materials Wiley Online Library
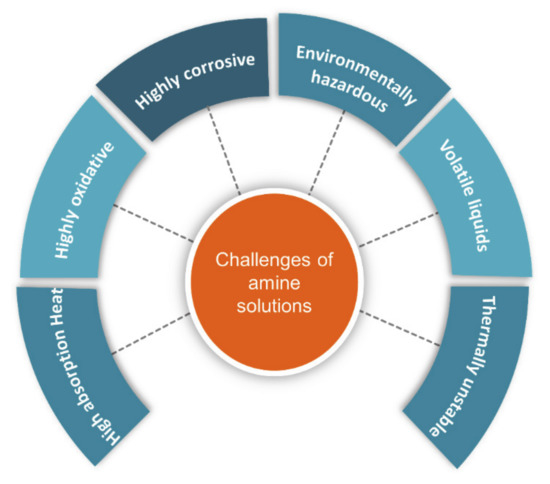
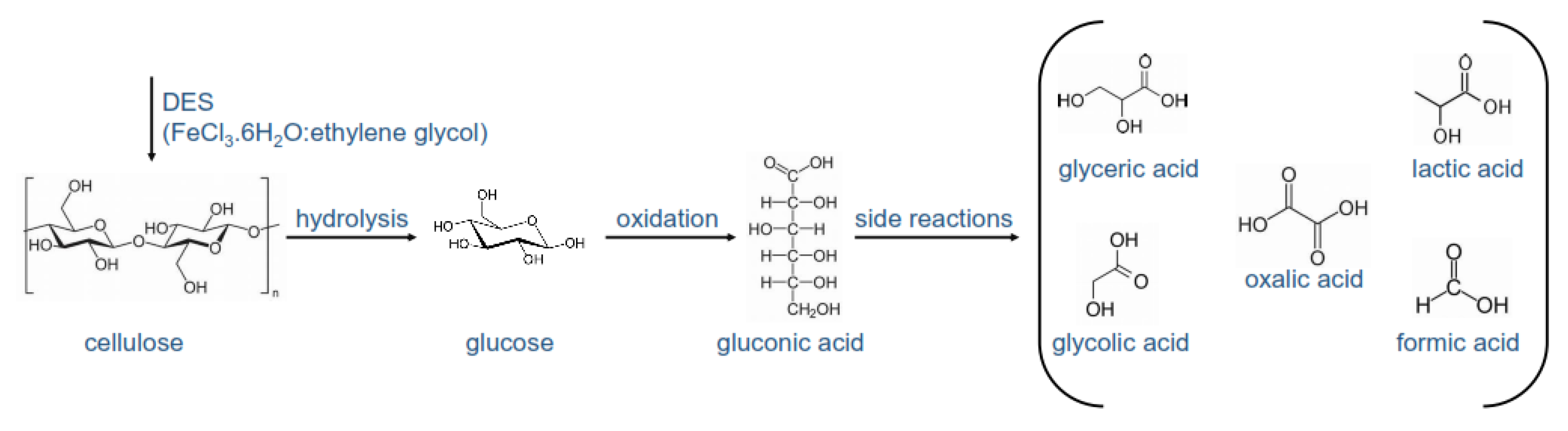
Molecules Free Full Text Utilization Of Deep Eutectic Solvents To Reduce The Release Of Hazardous Gases To The Atmosphere A Critical Review Html

The Following Energy Diagram Depicts A Reaction Where An Alcohol Is Converted To An Alkyl H Oneclass
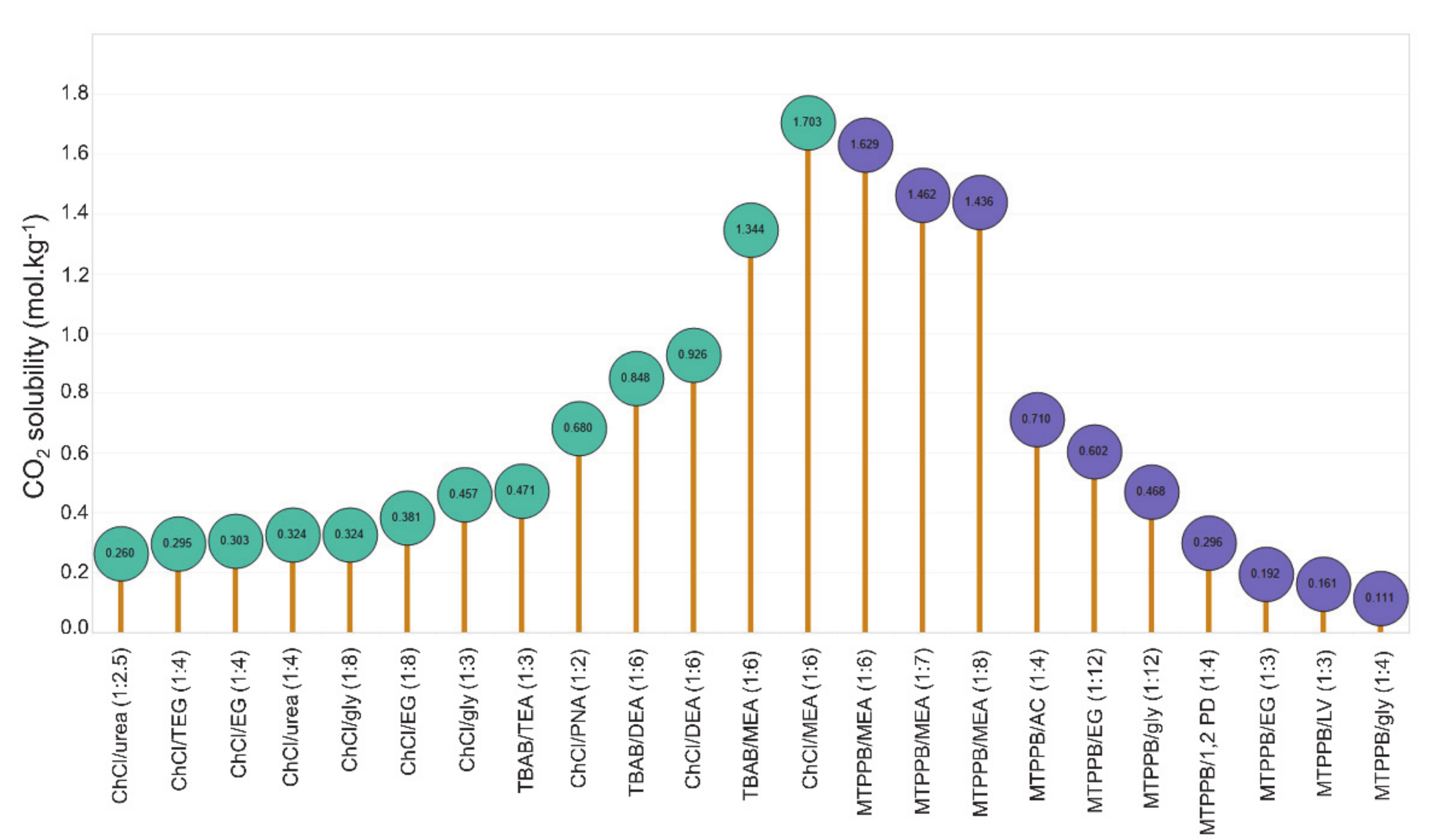
Molecules Free Full Text Utilization Of Deep Eutectic Solvents To Reduce The Release Of Hazardous Gases To The Atmosphere A Critical Review Html

Advances In Oxosulfonylation Reaction Ghosh 2020 Advanced Synthesis Amp Catalysis Wiley Online Library











0 Response to "38 the following energy diagram depicts a reaction where an alcohol is converted to an alkyl halide"
Post a Comment