38 potential energy diagram for endothermic reaction
The starting materials of the reaction. C. A local maximum on the energy diagram. D. A low-energy point between the starting materials and the product. 20. Which of the following energy. Question: 18. Draw an energy diagram for an endothermic reaction with two steps reaction coordinate 19. ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
9 Jul 2019 — A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the ...
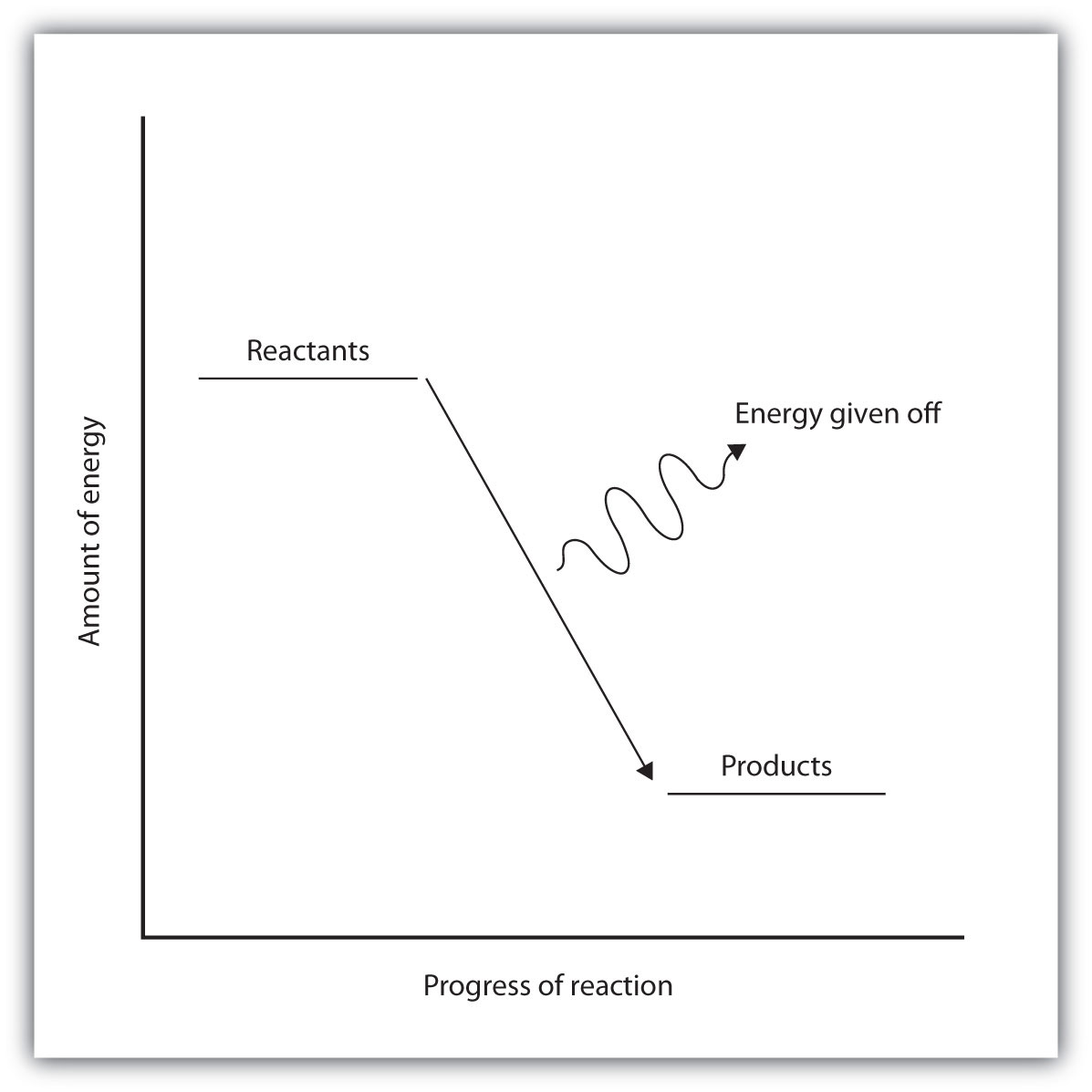
Potential energy diagram for endothermic reaction
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ... A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ... 9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Potential energy diagram for endothermic reaction. This reaction is the combustion of propane (C 3 H 8). Combustion is a reaction where a compound burns in oxygen to produce carbon dioxide (CO 2) and water (H 2 O). To draw the potential energy diagram for this reaction, we have to first determine whether this reaction is endothermic or exothermic. energy needed for a reaction to happen - this lower activation energy is shown by the dotted red line in the diagram here. Energy level diagrams for endothermic reactions In endothermic reactions the reactants have a less energy than the products . The difference between these two energy levels is the energy absorbed from the surroundings. An endothermic reaction must have a positive change in enthalpy. That is, Delta"H">0. This means that the system absorbs heat. To represent the positive change in enthalpy, the potential energy of the reactants must be LOWER than that of the products. Notice how the end of the "hump" is higher than the beginning, showing how the system gained energy during the course of the reaction. Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Energy reactants products exothermic reactions the reactants have more potential energy than the ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ... A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ... Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...


0 Response to "38 potential energy diagram for endothermic reaction"
Post a Comment